argon valence shell|1.9B: Valence and Core Electrons : Pilipinas Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p subshell. We can write the configuration of oxygen's valence electrons as 2 . Total Eclipse of the Fart: Directed by George Elliott, Keith Oliver. With Rob Tinkler, Bahia Watson, Wyatt White. Leshawna hatches a plan to extort the tooth fairy for money after destroying her loose tooth, .
PH0 · Valences of the Chemical Elements
PH1 · Valence electrons and ionic compounds (video)
PH2 · Valence electrons (video)
PH3 · Valence electron
PH4 · Valence Electrons Chart for All Elements
PH5 · The periodic table, electron shells, and orbitals
PH6 · How Many Valence Electrons Does Argon (Ar) Have? [Valency of Ar]
PH7 · How Many Valence Electrons Does Argon (Ar) Have?
PH8 · 3.7: Electrons and Valence Shells
PH9 · 3.10: Valence Electrons
PH10 · 1.9B: Valence and Core Electrons
There are 9 or 18 rounds of Card Golf, just like the 9 or 18 holes of Golf. Card Golf utilizes a scorecard that is reminiscent of a scoresheet from traditional Golf, and it also utilizes a penalty points system, where the lower score is better, just like in the traditional game.
argon valence shell*******Mar 23, 2023
Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2 s subshell and four in the 2 p subshell. We can write the configuration of oxygen's valence electrons as 2 .1.9B: Valence and Core Electrons The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as .
argon valence shell You may assume the valences of the chemical elements—the number of . It's not unreasonable for calcium to lose two electrons to have a stable outer shell, to have an electron configuration like argon. So if it loses two electrons it has a positive two charge. And you could imagine, those two electrons get lost to two different .
Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy .Valence shell. The valence shell is the set of orbitals which are energetically accessible for accepting electrons to form chemical bonds. For main-group elements, the valence shell .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called . Noble gases like argon have eight valence electrons so it does not require to lose or gain electrons to complete its energy shell i.e. stable octet. So that they do not have any tendency to combine with .Element Argon (Ar), Group 18, Atomic Number 18, p-block, Mass 39.95. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition .

Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition .
Electrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency (or valence) of an atom. . Valency of Chlorine: 17: 1: .
Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
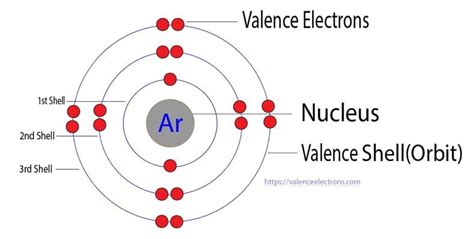
The noble gases (Group 18) are located in the far right of the periodic table and were previously referred to as the "inert gases" due to the fact that their filled valence shells (octets) make them extremely nonreactive. The noble gases were characterized relatively late compared to other element groups. Argon, chemical element, inert gas of Group 18 (noble gases) of the periodic table, terrestrially the most abundant and industrially the most frequently used of the noble gases. It is used in gas-filled electric light bulbs, radio tubes, and Geiger counters. . The outermost (valence) shell of argon has eight electrons, making it exceedingly . Explanation: Argon is a noble gas with an electron configuration of [N e]3s23p6. So, it has 3 electron shells, and that will be its valence shell. In the third shell, it holds a total of 2 + 6 = 8 electrons, so it has 8 valence electrons. Answer link. 8 Argon is a noble gas with an electron configuration of [Ne]3s^2\3p^6.
argon valence shell 1.9B: Valence and Core Electrons This means the first shell (1s) has 2 electrons. The second shell (2s and 2p) has a total of 8 electrons. And, the third (last) shell has 1 electron. The first and second shells comprise the core (inner) electrons = 2 + 8 = 10 electrons. The outermost (valence) has 1 electron. The shell diagram of the Na atom is shown here.
New Mexico Sunshine Laws. Inspection of Public Records Act; Open Meetings Act; Charities. Register a Charity Online; . Tobacco Manufacturers Directory; Portals. Missing and Murdered Indigenous People; REACH App; How do I get information on registered charities? Facebook Instagram Linkedin Youtube Twitter. 408 Galisteo Street. Villagra .Watch Pinay tulog tinira Free porn videos. You will always find some best Pinay tulog tinira videos xxx.
argon valence shell|1.9B: Valence and Core Electrons